04 Jun Electrochemistry
Electrochemistry is a branch of chemistry that studies the reactions resulting from the combined chemical and electrical effects. This type of chemistry deals with the production of electricity from the energy released during spontaneous chemical reactions. The reactions involve electron transfer, so they are oxidation-reduction reactions. Many metals are purified or electroplated using electrochemical methods. Devices such as automobiles, smartphones, watches, pacemakers, etc use batteries for power. In batteries, chemical reactions occur which produce electricity spontaneously and convert them into useful work. Students are explained the concept of electrochemistry from secondary school. Chemistry tuition explains why electrochemistry is important and uses examples to give students a better understanding of the topic.
This form of study covers electrolytic processes and galvanic or voltaic processes. Electrolytic process is the one in which chemical changes occur in the area of an electrical current. Galvanic processes are chemical reactions resulting in the production of electrical energy.
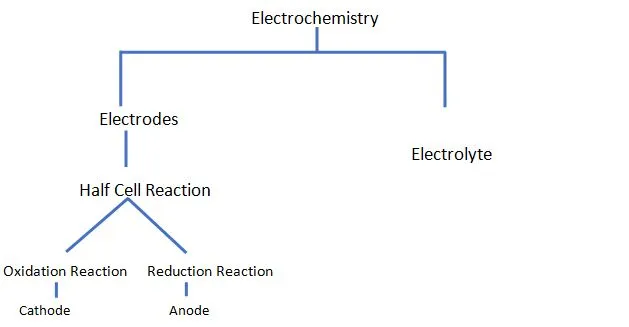
An electrochemical cell consists of two electronic conductors (electrodes) and an ionic conductor (electrolyte). An electrolytic cell is an electrochemical cell in which the energy is derived from an external power source to create a non- spontaneous reaction.
A half- cell reaction is when an electrochemical reaction occurs at each electrode. The reaction is termed as half-cell reaction since there are two electrodes in a single cell at which the reaction takes place. The overall chemical reaction is obtained by merging two individual half cell reactions. Half cell reactions are of two types: Oxidation Reactions and Reduction Reaction.
Depending on the direction of electron transfer, a reaction is classified as oxidation or reduction reaction. An oxidation reaction includes the loss of an electron. It is the transfer of electrons from the species to the electrode. A reduction reaction, on the other hand, includes the gain of an electron. It involves the transfer from an electrode to a species. An electrode in which the oxidation reaction is inhibited is called a cathode and an anode is an electrode in which the reduction reaction takes place.
If you face difficulties in Chemistry and feel sad or shy about it then we will ask you to stop thinking in that way. It is perfectly normal to face difficulties with a subject which other students in your class may not feel the same way. It takes gut to be different. Come to us at Miracle Learning Centre where we are different than any other education center of Singapore. We teach students with fun, we make them self sufficient and make them be successful in their own careers. Offering secondary science tuition, primary science tuition, ip science tuition from some of the best teachers of Singapore.

